Oligomerization of phosphaalkynes mediated by bulky N-heterocyclic carbenes: avenues to novel phosphorus frameworks†
Abstract
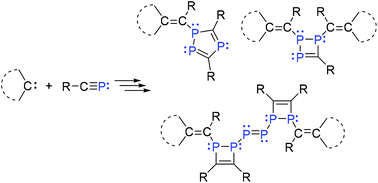
Reactions of RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (R = tBu or Ad (admantyl)) with NHCs (SIMes, 1a; IMes, 1b and IDipp, 1d), leading to 1,2,3-triphosphetenes 2 and 3, a triphosphole 4, and a di-1,2-dihydro-1,2-diphosphete-substituted diphosphene 5, are reported. Compound 5 represents a novel P6 chain framework, as well as a newly discovered phosphaalkyne hexamer stabilized by two NHCs. Computational investigations suggest that the weak π-accepting ability of NHCs results in low stability of the initially formed 2H-phosphirenes, thus facilitating spontaneous oligomerization reactions. Such oligomerizations are highly susceptible to the electronic property and steric bulk of NHCs.
P (R = tBu or Ad (admantyl)) with NHCs (SIMes, 1a; IMes, 1b and IDipp, 1d), leading to 1,2,3-triphosphetenes 2 and 3, a triphosphole 4, and a di-1,2-dihydro-1,2-diphosphete-substituted diphosphene 5, are reported. Compound 5 represents a novel P6 chain framework, as well as a newly discovered phosphaalkyne hexamer stabilized by two NHCs. Computational investigations suggest that the weak π-accepting ability of NHCs results in low stability of the initially formed 2H-phosphirenes, thus facilitating spontaneous oligomerization reactions. Such oligomerizations are highly susceptible to the electronic property and steric bulk of NHCs.


 Please wait while we load your content...
Please wait while we load your content...