Perovzalates: a family of perovskite-related oxalates†
Abstract
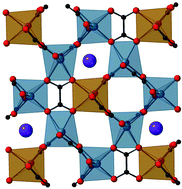
A family of hybrid Perovskite-oxalates (“Perovzalates”) of general composition AILi3MII(C2O4)3 (A = K+, Rb+, Cs+; M = Fe2+, Co2+, Ni2+) are presented. All eight new compounds are isostructural with the previously reported examples NH4Li3Fe(C2O4)3 and KLi3Fe(C2O4)3, crystallising in the rhombohedral space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, with a ∼11.3–11.6 Å, c ∼14.8–15.2 Å. In contrast to other families of “hybrid perovskites” such as the formates, these compounds can be regarded as closer structural relatives to inorganic (oxide) perovskites, in the sense that they contain direct linkages of the octahedral sites via bridging oxygen atoms (of the oxalate groups). It is of note, therefore, that monoatomic cations as large as Cs+ can be incorporated into the perovskite-like A sites of this structure type, which is not feasible in traditional ABO3 perovskites; indeed CsLi3Ni(C2O4)3 appears to exhibit the ‘mostly tightly bound’ 12-coordinate Cs+ ion in an oxide environment, according to a bond valence analysis.
c, with a ∼11.3–11.6 Å, c ∼14.8–15.2 Å. In contrast to other families of “hybrid perovskites” such as the formates, these compounds can be regarded as closer structural relatives to inorganic (oxide) perovskites, in the sense that they contain direct linkages of the octahedral sites via bridging oxygen atoms (of the oxalate groups). It is of note, therefore, that monoatomic cations as large as Cs+ can be incorporated into the perovskite-like A sites of this structure type, which is not feasible in traditional ABO3 perovskites; indeed CsLi3Ni(C2O4)3 appears to exhibit the ‘mostly tightly bound’ 12-coordinate Cs+ ion in an oxide environment, according to a bond valence analysis.


 Please wait while we load your content...
Please wait while we load your content...