Structural insights into three phosphates with distinct polyanionic configurations†
Abstract
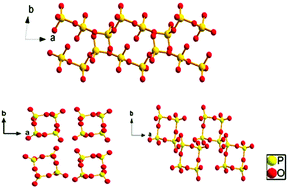
Three mixed-metal phosphates, namely, centrosymmetric LiSrP3O9 and LiCsP2O6 as well as noncentrosymmetric K2SrP4O12 have been synthesized by the high temperature solution method. These structures were characterized by single-crystal X-ray diffraction and their P–O units are shown to feature distinct polyanionic configurations from [PO3]∞ chains in LiSrP3O9 to [P4O12]4− rings in LiCsP2O6 and K2SrP4O12. Besides, K2SrP4O12 shows a moderate second harmonic generation response about 0.6 times that of KH2PO4 and can be applied as a potential nonlinear optical material for the ultraviolet spectral region. Thermal behaviour analyses indicate that the compounds LiSrP3O9 and K2SrP4O12 remain stable up to 659 and 698 °C, respectively. In addition, the crystal structures, thermal stabilities and infrared spectra of the title compounds have been examined.


 Please wait while we load your content...
Please wait while we load your content...