Copper(ii) complexes with tridentate Schiff base-like ligands: solid state and solution structures and anticancer activity†
Abstract
We report 15 new Cu(II) complexes with tridentate NNO β-acylenamino ligands derived from 2-picolylamine and bearing up to three alkyl, alkoxy, alkoxycarbonyl, or (pseudo)halide substituents. The structures of nine complexes were elucidated by single crystal X-ray diffraction analysis. Complexes with an unsubstituted pyridine ring crystallised with a square pyramidal coordination sphere, whereas substitution of the pyridine ring led to a square planar coordination sphere around the metal centre. The solution structures and properties of the complexes were characterised by UV-Vis spectroscopy and cyclic voltammetry. They were also tested for their cytotoxic effect on four human cancer cell lines. Two complexes were identified that were highly active with single-digit IC50 values, exceeding those of cisplatin by far. A tentative structure–activity relationship was proposed as well as topoisomerase I inhibition as a possible mode of action, while any significant interference with DNA and the level of reactive oxygen species could be excluded.
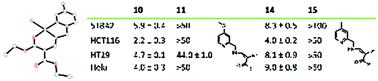


 Please wait while we load your content...
Please wait while we load your content...