Elucidating the origins of enhanced CO2 reduction in manganese electrocatalysts bearing pendant hydrogen-bond donors†
Abstract
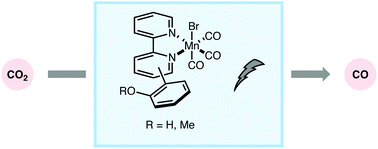
Complexes of the general form [Mn(X)(CO)3bpy] (X = a variety of monodentate ligands, bpy = 2,2′-bipyridine) have been reported to act as electrocatalysts for the reduction of CO2 to CO. In this work, a series of phenol and anisole substituted bipyridine ligands were synthesized and ligated to a manganese metal center in order to probe for an intramolecular hydrogen-bonding interaction in the transition state of CO2 reduction. Ligands without the ability to intramolecularly hydrogen bond displayed decreased catalytic current density compared to those with the ability to hydrogen bond with CO2. Electrocatalysis was studied by performing voltammetric and bulk electrolysis experiments under argon or CO2 environments. Measurements of catalytic rates using hydrogen vs. deuterium for the intramolecular H/D-bonding step show that there is an isotope effect associated with the catalysis. The data presented herein suggest a mechanism involving two subsequent equilibrium isotope effects in combination with a primary kinetic isotope effect.


 Please wait while we load your content...
Please wait while we load your content...
