1,5-Naphthyl-linked bis(imino)pyridines as binucleating scaffolds for dicobalt ethylene oligo-/polymerization catalysts: exploring temperature and steric effects†
Abstract
Six examples of dinuclear bis(imino)pyridine-cobalt(II) complexes, [1,5-{2-(CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)-6-(CMe
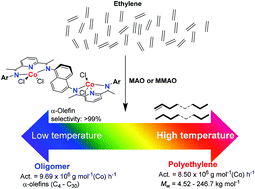
N)-6-(CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(2,6-R12-4-R2-C6H2))C5H3N}2(C10H6)]Co2Cl4 (R1 = Me, R2 = H Co1; R1 = Et, R2 = H Co2; R1 = iPr, R2 = H Co3; R1 = Me, R2 = Me Co4; R1 = Et, R2 = Me Co5; R1 = CHPh2, R2 = Me Co6), have been prepared from the corresponding bis(tridentate) compartmental ligands (L1–L6) in reasonable yields. The molecular structures of Co3 and Co5 revealed two N,N,N-cobalt dichloride units to adopt anti-positions about the 1,5-naphthyl linking unit, with each cobalt center exhibiting a distorted trigonal bipyramidal geometry. On activation with either MAO or MMAO, Co1–Co6 were shown to promote both polymerization and oligomerization of ethylene with high overall activities (up to 1.03 × 107 gPE per·mol(Co) per·h for Co1/MAO at 70 °C). Curiously, on increasing the reaction temperature a larger proportion of polymer was noted, while at lower temperature an enhanced selectivity for oligomer was seen. In general, the oligomeric products displayed Schulz–Flory distributions with high selectivities for α-olefins (>99%). On the other hand, the highly linear polymers displayed narrow dispersities and comprised both fully saturated and unsaturated chain ends with the vinyl content (–CH
N(2,6-R12-4-R2-C6H2))C5H3N}2(C10H6)]Co2Cl4 (R1 = Me, R2 = H Co1; R1 = Et, R2 = H Co2; R1 = iPr, R2 = H Co3; R1 = Me, R2 = Me Co4; R1 = Et, R2 = Me Co5; R1 = CHPh2, R2 = Me Co6), have been prepared from the corresponding bis(tridentate) compartmental ligands (L1–L6) in reasonable yields. The molecular structures of Co3 and Co5 revealed two N,N,N-cobalt dichloride units to adopt anti-positions about the 1,5-naphthyl linking unit, with each cobalt center exhibiting a distorted trigonal bipyramidal geometry. On activation with either MAO or MMAO, Co1–Co6 were shown to promote both polymerization and oligomerization of ethylene with high overall activities (up to 1.03 × 107 gPE per·mol(Co) per·h for Co1/MAO at 70 °C). Curiously, on increasing the reaction temperature a larger proportion of polymer was noted, while at lower temperature an enhanced selectivity for oligomer was seen. In general, the oligomeric products displayed Schulz–Flory distributions with high selectivities for α-olefins (>99%). On the other hand, the highly linear polymers displayed narrow dispersities and comprised both fully saturated and unsaturated chain ends with the vinyl content (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) found to rise with the reaction temperature. By modulating the steric hindrance exerted by the ortho-R1 substituents in the precatalyst, polyethylenes displaying a remarkably broad range of molecular weights could be obtained [from 4.52 kg mol−1 (R1 = Me) to 246.7 kg mol−1 (R1 = CHPh2)].
CH2) found to rise with the reaction temperature. By modulating the steric hindrance exerted by the ortho-R1 substituents in the precatalyst, polyethylenes displaying a remarkably broad range of molecular weights could be obtained [from 4.52 kg mol−1 (R1 = Me) to 246.7 kg mol−1 (R1 = CHPh2)].


 Please wait while we load your content...
Please wait while we load your content...