Solvent-assisted ligand exchange (SALE) for the enhancement of epoxide ring-opening reaction catalysis based on three amide-functionalized metal–organic frameworks†
Abstract
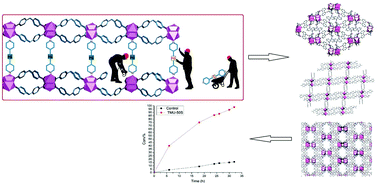
In recent years, functionalized pillar ligands have gained significant interests due to their important role in MOF structure and performance. The synthesis of MOF compounds with a particular functionalized ligand is not always successful, and sometimes, synthesis cannot be achieved easily or directly, even by employing several methods. However, this limitation can be overcome by applying a post-synthesis step that swaps the functional groups without changing the backbone of the pillar ligand. Solvent-assisted ligand exchange (SALE) is a post-synthesis method that has been used for confronting this challenge by replacing a functional group with an alternative. Through this investigation, we tried to improve the properties of MOF compounds and increase their catalytic efficiency by importing new functional groups into their structures. The N1,N3-di (pyridine-4-yl) malonamide linker (S) is a pillar ligand, which does not easily enter into the structure during the synthesis of MOF compounds. Therefore, to solve this issue, amide-functionalized, benzene-core ligand derivatives were designed as linkers to manufacture the new 3D structures [Co(oba)(bpta)]·(DMF)2 TMU-50 and [Co2(oba)2(bpfn)]·(DMF)2.5 TMU-51 and the novel 2D structure [Co(oba)(bpfb)]·(DMF)2 TMU-49. These structures were achieved by layering the compounds via hydrothermal reaction. Moreover, the ability of these structures to act as catalysts was evaluated using the methanolysis reaction of epoxides. To increase the MOF catalytic efficiency, we designed the N1,N3-di (pyridine-4-yl) malonamide linker (S) as a malonamide pillar ligand, which contains an acidic hydrogen that is suitable for catalyzing an epoxide ring-opening reaction and therefore enhancing the catalytic activity. As the synthesis of the MOF structure with this linker was not successful, we designed three new structures by incorporating different percentages of S linkers by exchanging the acylamide functional group with malonamide via the SALE pathway. The acylamide functional group was successfully replaced and produced daughter MOFs TMU-49S, TMU-50S and TMU-51S. PXRD and NMR spectroscopy confirmed that the S linker was incorporated into the acylamide-MOF structure. The obtained materials TMU-49S, TMU-50S and TMU-51S are isostructural with their parent frameworks. The S spacer significantly improved the catalytic properties of the MOF compounds in the ring-opening reaction of epoxides, with TMU-50S showing a 98% catalytic efficiency after incorporating the S linker. The catalysts could be recycled without any significant loss in the catalytic efficiency.


 Please wait while we load your content...
Please wait while we load your content...