How does Mo-dependent perchlorate reductase work in the decomposition of oxyanions?†
Abstract
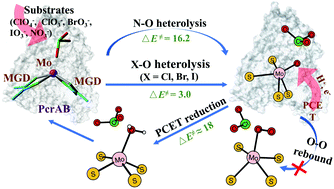
Mo-Dependent perchlorate reductase (PcrAB), responsible for the ending step of anaerobic respiration of perchlorate reducing bacteria, catalyzes the conversion of perchlorate to chlorate and subsequently chlorite and is also able to deoxidate bromate, iodate, and nitrate. Herein, the reaction mechanisms for the PcrAB-catalyzed decomposition of these oxyanions have been investigated using density functional calculations and a chemical model constructed from the X-ray crystal structure. It is revealed that the reactions of halogen oxyanions proceed through a very fast O–X (X = Cl, Br, and I) heterolytic cleavage activated by the MoIV center, followed by a rate-limiting reduction of the resulting MoVI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O back to MoIV dominated by the slow proton-coupled electron transfer (PCET). However, the O–N bond heterolysis in the nitrate decomposition has a barrier (16.2 kcal mol−1) comparable to the PCET-dominating reduction of MoVI
O back to MoIV dominated by the slow proton-coupled electron transfer (PCET). However, the O–N bond heterolysis in the nitrate decomposition has a barrier (16.2 kcal mol−1) comparable to the PCET-dominating reduction of MoVI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. This heralds an exciting future where a proper mutation of electron/proton transfer passage of perchlorate reducing bacteria may lead to a decomposition preference for halogen oxyanions rather than non-toxic nitrate, providing a friendly bioremediation method. Other open mechanistic questions are also addressed, where in particular an O–O rebound mechanism without PCET has been ruled out.
O. This heralds an exciting future where a proper mutation of electron/proton transfer passage of perchlorate reducing bacteria may lead to a decomposition preference for halogen oxyanions rather than non-toxic nitrate, providing a friendly bioremediation method. Other open mechanistic questions are also addressed, where in particular an O–O rebound mechanism without PCET has been ruled out.


 Please wait while we load your content...
Please wait while we load your content...