Tellurorhodamine photocatalyzed aerobic oxidation of organo-silanes and phosphines by visible-light†
Abstract
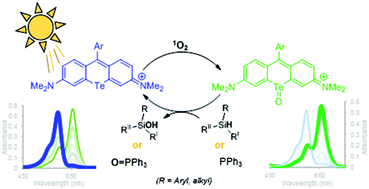
Tellurorhodamine, 9-mesityl-3,6-bis(dimethylamino)telluroxanthylium hexafluorophosphate (1), photocatalytically oxidizes aromatic and aliphatic silanes and triphenyl phosphine under mild aerobic conditions. Under irradiation with visible light, 1 can react with self-sensitized 1O2 to generate the active telluroxide oxidant (2). Silanes are oxidized to silanols and triphenyl phosphine is oxidized to triphenyl phoshine oxide either using 2, or 1 with aerobic irradiation. Kinetic experiments coupled with a computational study elucidate possible mechanisms of oxidation for both silane and phosphine substrates. First-order rates were observed in the oxidation of triphenyl phosphine and methyldiphenyl silane, indicating a substitution like mechanism for substrate binding to the oxidized tellurium(IV). Additionally, these reactions exhibited a rate-dependence on water. Oxidations were typically run in 50 : 50 water/methanol, however, the absence of water decreased the rates of silane oxidation to a greater degree than triphenyl phosphine oxidation. Parallel results were observed in solvent kinetic isotope experiments using D2O in the solvent mixture. The rates of oxidation were slowed to a greater degree in silane oxidation by 2 (kH/kD = 17.30) than for phosphine (kH/kD = 6.20). Various silanes and triphenyl phosphine were photocatalytically oxidized with 1 (5%) under irradiation with warm white LEDs using atmospheric oxygen as the terminal oxidant.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...
