A supramolecular assembly bearing an organic TADF chromophore: synthesis, characterization and light-driven cooperative acceptorless dehydrogenation of secondary amines†
Abstract
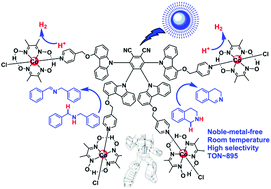
A noble-metal-free chromophore–catalyst supramolecular assembly, which is composed of an organic thermally activated delayed fluorescence (TADF) chromophore and cobaloximes, has been designed and synthesized for the first time for the efficient acceptorless dehydrogenation of secondary amines under blue light irradiation at room temperature. The TADF chromophore has a long lifetime of 17.4 μs with suitable redox potentials for the selective acceptorless dehydrogenation of secondary amines to afford imines and H2 through cooperative catalysis of the chromophore and cobaloximes in the supramolecular assembly. A high TON of 895 was obtained for the acceptorless dehydrogenation of 1,2,3,4-tetrahydroisoquinoline despite its high oxidation potential (+1.38 V vs. SCE).


 Please wait while we load your content...
Please wait while we load your content...