Enhanced photocatalytic hydrogen evolution over bimetallic zeolite imidazole framework-encapsulated CdS nanorods†
Abstract
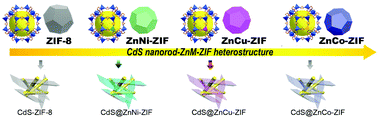
A hybrid of ZIF-8 with CdS nanorods could increase the transport efficiency of photo-generated charge carriers and the surface area. Notably, through doping Zn ions with a transition metal, in this work, we fabricated a bimetallic ZnM-ZIF (M = Ni, Cu, or Co)-encapsulated CdS nanorod heterostructure for the first time. Compared with ZIF-8, the bimetallic ZIF exhibited a modulated structure, flat band position, and lower overpotential for the hydrogen evolution reaction. ZnM-ZIF not only improved the transfer of water and light, but also boosted the separation of charge carriers. Consequently, the optimized CdS-ZnM-ZIF samples with Cu, Ni, and Co doping showed corresponding photocatalytic hydrogen activities 44, 92, and 59 times larger than that of pristine CdS nanorods. This work provides a new method for better utilization of porous MOF crystals for photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...