Isolation of two bis(silyl)nickel complexes with Si–Si bond formation in a single-crystal-to-single-crystal fashion†
Abstract
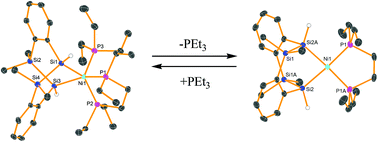
A unique penta-coordinate nickel complex [{1,2-C6H4(SiMe2)(SiH)}2Ni(PEt3)(depe)] (6) that generates two new Si–Si single bonds has been prepared exclusively by the reaction of a chelating silyl hydride 1-(dimethylsilyl)-2-silylbenzene (4) and Ni(depe)(PEt3)2 (depe = Et2PCH2CH2PEt2) in a 2 : 1 ratio. Complex 6 is the first example of mononuclear silyl nickel complexes containing a Si–Si single bond formed in situ. Interestingly, framework 6 exhibits reversible single-crystal-to-single-crystal transformations upon the removal and rebinding of the coordinating PEt3 molecule, which give rise to a PEt3-free complex [{1,2-C6H4(SiMe2)(SiH)}2Ni(depe)] (6′), concomitant with the alteration of the coordination geometry of central metal atoms.


 Please wait while we load your content...
Please wait while we load your content...