Cation sensing by luminescent high-nuclearity Zn–Eu Schiff base nanoscale complexes: high sensitivity to Ag+ and Cd2+ ions at the ppm level†
Abstract
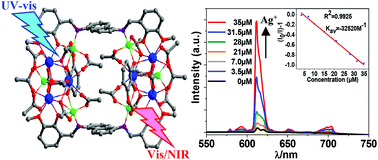
Three polynuclear Zn–Ln nanoscale complexes [Ln2Zn2(L1)2(OAc)6] (Ln = Eu (1) and Nd (2)) and [Eu6Zn6(L2)2(L3)2O2(OAc)18] (3) were constructed using two Schiff base ligands featuring Ph(CH2)Ph (H2L1) and naphthyl (H2L2) backbones. The use of the Schiff base ligand H2L1 leads to the formation of linear complexes 1 and 2 with molecular sizes of approximately 7 × 10 × 24 Å, while planar complex 3 (approximately 10 × 15 × 22 Å) is constructed from H2L2. In 1–3, the chromogenic Zn/ligand moieties can absorb energy from and transfer energy to lanthanide centers, and all of these complexes display the typical emissions of Ln3+ ions. Interestingly, 3 displays luminescence response to the addition of metal ions and has high sensitivity to Ag+ and Cd2+ ions at the ppm level.


 Please wait while we load your content...
Please wait while we load your content...