Highly efficient metal(iii) porphyrin and salen complexes for the polymerization of rac-lactide under ambient conditions†
Abstract
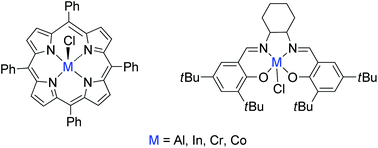
Ligated metal(III) complexes, Al(III), In(III), Cr(III), and Co(III), containing tetraphenylporphyrin (TPP) and cyclohexylsalen (Cy-salen) ligands were investigated for the polymerization of rac-lactide (rac-LA). A combination of metal complexes, co-catalyst, and co-initiator was shown to be highly efficient for the ring-opening polymerization of rac-LA at room temperature. Influences of metal centers, ligands, and co-catalysts were investigated. Higher Lewis acidity of metal centers and ligands enhanced catalytic activity. Ionic co-catalysts showed increased polymerization rates. Polymerization of rac-LA catalyzed by tetraphenylporphyrin aluminum chloride ((TPP)AlCl) and bis(triphenylphosphine)iminium chloride (PPN+Cl−) in cyclohexene oxide (CHO) as a solvent produced isotactic-enriched polylactide. The order of reactivities based on the metals supported by the TPP ligand is Cr(III) > Al(III) > In(III) > Co(III) while the reactivities of the catalysts supported by Cy-salen ligands are in the order In(III) > Cr(III) > Al(III) > Co(III).


 Please wait while we load your content...
Please wait while we load your content...