A water-stable europium-MOF as a multifunctional luminescent sensor for some trivalent metal ions (Fe3+, Cr3+, Al3+), PO43− ions, and nitroaromatic explosives†
Abstract
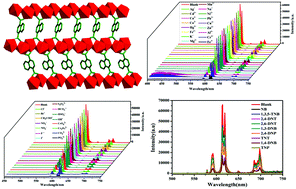
A luminescent Eu-MOF, namely, [Eu2(ppda)2(npdc)(H2O)]·H2O (1) based on the mixed ligands 4-(pyridin-3-yloxy)-phthalic acid (H2ppda) and naphthalene-1,4-dicarboxylic acid (H2npdc) was synthesized under solvothermal conditions. Compound 1 was initially constructed from the 1D binuclear chains of [Ln(npdc)]2 and further connected by ppda2− ligands, forming a 2D layered structure. It is worth noting that compound 1 exhibited excellent fluorescence stability in the pH range of 2–13 in an aqueous solution. It was found that compound 1 could not only detect trivalent Fe3+, Cr3+ and Al3+ ions with high selectivity and recyclability but also serve as an excellent selective sensing material for PO43− ions among some anions. The detection of NACs demonstrated that compound 1 could also behave as a functional probe with high selectivity, sensitivity, and recyclability, and the detection limit of TNP was 2.97 × 10−6 M. Furthermore, the luminescent sensing mechanisms for different analytes were speculated.


 Please wait while we load your content...
Please wait while we load your content...