Achieving nitrogen-doped carbon/MnO2 nanocomposites for catalyzing the oxygen reduction reaction†
Abstract
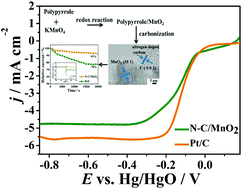
The design and synthesis of cheaper and more stable catalysts with comparable electrocatalytic performance to commercial Pt/C towards the oxygen reduction reaction is of importance for their application in fuel cells and metal–air batteries. Herein, we report the generation of nitrogen-doped carbon/MnO2 nanocomposites via pyrolyzing the polypyrrole/MnO2 precursor that is obtained based on the direct redox reaction between polypyrrole and KMnO4. The achieved sample presents an onset and half-wave potential of −0.048 and −0.124 V vs. Hg/HgO, respectively, and a current retention of 93% after 20 000 s chronoamperometry measurement. Meanwhile, it shows stronger methanol tolerance, endowing it with great potential for the corresponding applications. The excellent oxygen reduction reaction catalytic activity originates from the synergistic effect between nitrogen-doped carbon and MnO2, where the former one is helpful for MnO2 to achieve a higher Mn3+/Mn4+ ratio and a higher number of oxygen vacancies, and the existence of the latter one is beneficial for generating a higher ID/IG ratio in the carbon layer. These both contribute to the achievement of large numbers of active sites for the electrocatalytic process.


 Please wait while we load your content...
Please wait while we load your content...