Post synthetic exchange enables orthogonal click chemistry in a metal organic framework†
Abstract
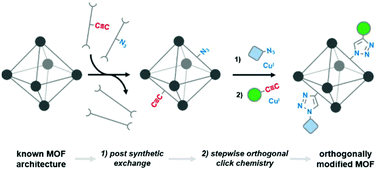
Biphenyl-4,4′-dicarboxylic acid derivatives containing either azide or acetylene functional groups were inserted into UiO-67 metal organic frameworks (MOFs) via post synthetic linker exchange. Sequential and orthogonal click reactions could be performed on these modified MOFs by incubating the crystals with small molecule substrates bearing azide or acetylene groups in the presence of a copper catalyst. 1H NMR of digested MOF samples showed that up to 50% of the incorporated linkers could be converted to their “clicked” triazole products. Powder X-ray diffraction confirmed that the UiO-67 structure was maintained throughout all transformations. The click reaction efficiency is discussed in context of MOF crystallite size and pore size. As the incorporation of clicked linkers could be controlled by post synthetic exchange, this work introduces a powerful method of quickly introducing orthogonal modifications into known MOF architectures.


 Please wait while we load your content...
Please wait while we load your content...