Lewis acid activated CO2 reduction over a Ni modified Ni–Ge hydroxide driven by visible-infrared light†
Abstract
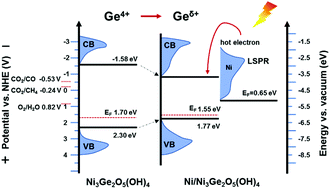
Improvement of light harvesting and reaction kinetics is of great importance for achieving efficient solar-driven CO2 reduction. Here, a Ni modified low-crystalline Ni–Ge containing hydroxide with Lewis acid sites was synthesized in highly reductive NaBH4 solution and exhibited 9.3 μmol gcat.−1 h−1 CO and 3.5 μmol gcat.−1 h−1 CH4 generation rates under visible light irradiation, and even achieved a 3.8 μmol gcat.−1 h−1 CO evolution under infrared light irradiation. The wide-spectrum light harvesting resulted from the light absorption from the localized surface plasmonic resonance of Ni nanoparticles. In addition, the Lewis acid can activate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds to decrease the kinetic barriers of CO2 reduction. The design concept that rationally combines the advantages of expanding the spectral response and activating CO2 may offer a new strategy for efficient solar energy utilization.
O bonds to decrease the kinetic barriers of CO2 reduction. The design concept that rationally combines the advantages of expanding the spectral response and activating CO2 may offer a new strategy for efficient solar energy utilization.


 Please wait while we load your content...
Please wait while we load your content...