Phenothiazines and phenoxazines: as electron transfer mediators for ferritin iron release†
Abstract
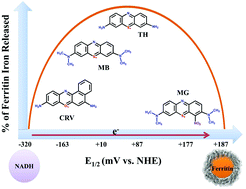
Intracellular ferritin stores iron as ferrihydrite and releases it for various cellular metabolic activities. The reductive approach, one of the possible mechanisms of iron mobilization from ferritin nanocages, requires electron transfer (ET) from reducing agent(s) to the protein encapsulated iron. In vitro, the rate of ET from the physiological reducing agent, NADH, to mineralized ferritin is very slow resulting in a smaller amount of iron release. Therefore, medically relevant phenothiazine (TH/MB/MG/TDB) and phenoxazine (BCB/CRV/NB) dyes were used as ET mediators to facilitate the electron relay and to evaluate their iron releasing ability from ferritin. These dyes have earlier been exploited as ET mediators during electrocatalysis and in the treatment of methemoglobinemia. With the exception of MG, the midpoint potentials (E1/2) and NADH oxidizing abilities of these dyes dictated by their structure and the reaction conditions along with the dye–ferritin interaction govern the kinetics of reductive iron mobilization. A greater amount of iron release was observed in the case of TH, BCB and CRV. In comparison to neutral pH, acidic pH altered E1/2 and protein conformation leading to enhanced iron mobilization, whereas dissolved O2 and the photosensitizing effect of dyes were found to have a negligible impact. In analogy to in vitro, the acidic environment of the lysosome may bring about similar changes in the reducing agents/dye mediators/ferritin to facilitate the iron release process in vivo. Following Marcus theory, our current observations suggest that the dyes with E1/2 values well separated from those of the reducing agents and ferritin's mineral core can be exploited to facilitate iron release during iron overload conditions.


 Please wait while we load your content...
Please wait while we load your content...