Strong electronic influence of equatorial ligands on frontier orbitals in paddlewheel dichromium(ii,ii) complexes†
Abstract
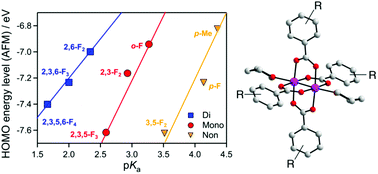
A series of substituted benzoate-bridged dichromium(II,II) complexes [Cr2(RCO2)4(THF)2] ([Cr2]), where RCO2− is substituted benzoate, was synthesized and its structural and magnetic properties were investigated. The orbital energies, as well as magnetic coupling energies, were also investigated using computational approaches. The HOMO/LUMO energy levels of the complexes are strongly dependent on the acidity, i.e., pKa, of the corresponding benzoic acids (RCO2H), revealing a linear trend in the respective groups of non-ortho, mono-ortho, and bi-ortho substituted groups. The Cr⋯Cr magnetic coupling constant (ES–T) is little affected by pKa; instead, the ES–T is associated with the HOMO/LUMO gap and strongly correlated with the Cr⋯Cr distance.


 Please wait while we load your content...
Please wait while we load your content...