Synthesis, catalysis, and DFT study of a ruthenium carbene complex bearing a 1,2-dicarbadodecaborane (12)-1,2-dithiolate ligand†
Abstract
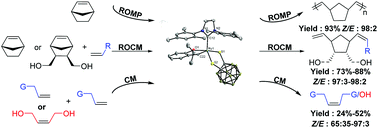
A ruthenium carbene catalyst containing a 1,2-dicarbadodecaborane(12)-1,2-dithiolate ligand was synthesized, and the structure was determined by single crystal X-ray diffraction. This new ruthenium carbene catalyst can catalyze the ring opening metathesis polymerization (ROMP) reaction of norbornene to give the corresponding Z-polymer (Z/E ratio, 98 : 2) in high yield (93%); ring opening cross metathesis (ROCM) reactions of norbornene/5-norbornene-2-exo, 3-exo-dimethanol with styrene or 4-fluorostyrene to give the corresponding Z-olefin products (Z/E ratios, 97 : 3–98 : 2), respectively, in high yields (73%–88%); cross metathesis (CM) reactions of terminal alkenes with (Z)-but-2-ene-1,4-diol to give high Z-olefin products in low yields; homometathesis reactions of terminal alkenes to give olefin products in low yields. Like other ruthenium carbene catalysts, the new complex tolerates many different functional groups. DFT calculations were also performed in order to understand the process of forming Z-olefin products and the decomposition process of catalysts.


 Please wait while we load your content...
Please wait while we load your content...