Solvent-induced formation of two gadolinium clusters demonstrating strong magnetocaloric effects and ferroelectric properties†
Abstract
Two gadolinium clusters, [Gd2(H3L)2(NO3)2](NO3)2 (1) and [Gd3(HL)(H2L)(NO3)4]·C2H5OH (2) (H4L = N,N,N′,N′-tetrakis(2-hydroxyethyl)ethylenediamine), were synthesized and characterized with different solvents. The magnetic properties of 1 and 2 were recorded and studied in detail. 1 is a centrosymmetric binuclear Gd2 cluster, adopting a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (point group
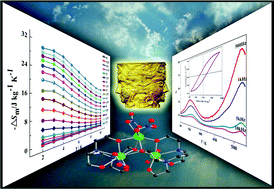
space group (point group ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), and exhibits a strong magnetocaloric effect (MCE) entropy with −ΔSm of 27.50 J kg−1 K−1. 2 crystallizes in the Pna21 space group (polar point group mm2) through solvent modulation and contains an asymmetric trinuclear Gd3 cluster. Magnetic and ferroelectric investigations of 2 reveal a large change in the magnetocaloric effect entropy with −ΔSm of 30.22 J kg−1 K−1, ferroelectric hysteresis and dielectric anomalies.
), and exhibits a strong magnetocaloric effect (MCE) entropy with −ΔSm of 27.50 J kg−1 K−1. 2 crystallizes in the Pna21 space group (polar point group mm2) through solvent modulation and contains an asymmetric trinuclear Gd3 cluster. Magnetic and ferroelectric investigations of 2 reveal a large change in the magnetocaloric effect entropy with −ΔSm of 30.22 J kg−1 K−1, ferroelectric hysteresis and dielectric anomalies.


 Please wait while we load your content...
Please wait while we load your content...