Bi2S3/C nanorods as efficient anode materials for lithium-ion batteries†
Abstract
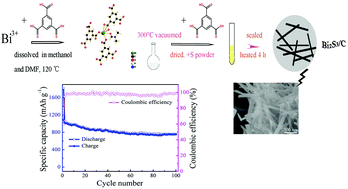
Bi2S3 is a promising negative electrode material for lithium storage owing to its high theoretical capacity. Nevertheless, the capacity of Bi2S3 decays very rapidly upon Li cycling. Here, Bi2S3 and Bi2S3/C were successfully synthesized by a novel route. Sulfur powder as a kind of sulfur source reacted with a metal organic framework based on bismuth and trimesinic acid—Bi(BTC)(DMF)·DMF·(CH3OH)2 (denoted as Bi-BTC). Trimesic acid further acted as a new type of carbon source to synthesize the Bi2S3/C composite. The particle sizes of the composite were smaller than those of pure Bi2S3, showing the suppression of Bi2S3 aggregation. Charge–discharge performance and cyclability for both the Bi2S3 and Bi2S3/C composites in lithium-ion batteries were measured. Specifically, the specific capacities of Bi2S3/C and Bi2S3 reached 765 and 603 mA h g−1, respectively, at 100 mA g−1 after 100 cycles. Because of its high capacity and excellent cycle life, Bi2S3/C is a promising energy storage material.


 Please wait while we load your content...
Please wait while we load your content...