Isolation of OH-bridged Ag(i)/Cu(iii) and ion-pair Cu(i)/Cu(iii) trifluoromethyl complexes with monophosphines†
Abstract
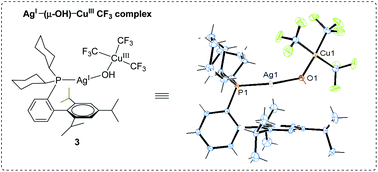
Cu(III)–CF3 complexes are important intermediates of both synthetic and mechanistic interest. This study describes the isolation, and spectroscopic and X-ray crystallographic characterization of CuIII–CF3 complexes 2–4 with typical monophosphine ligands PPh3 and Buchwald-type biarylmonophosphines. Distinct from the ion-pair [P2Cu(I)]+[Cu(III)(CF3)4]− structures of 2 and 4 (P: PPh3 or SPhos), complex 3 exhibits a novel OH-bridged Ag(I)–Cu(III) dinuclear structure with XPhos-coordinated linear Ag(I) and square planar Cu(III) components. This is the first heterobimetallic Cu(III)–CF3 complex confirmed by both solution-phase NMR spectroscopy and solid state X-ray crystal structure analysis. Complex 3 is found to have the LUMO orbital of major σ*(Cu–CF3) nature and electrophilic CF3 ligands. Accordingly, complex 3 is able to trifluoromethylate 2 equivalents of aryl boronic acids in up to quantitative yields, regardless of the inert or oxidative conditions. In contrast, the ion-pair complexes 2 and 4 show low reactivity. This study enriches the coordination and reactivity chemistry of Cu(III)–CF3 compounds and shows the feasibility of modulation of structures and reactivity by ligand design, which may inspire future efforts on Cu(III)–CF3 chemistry.


 Please wait while we load your content...
Please wait while we load your content...