Neptunium(iv)-hydroxamate complexes: their speciation, and kinetics and mechanism of hydrolysis
Abstract
Simple hydroxamic acids such as acteohydroxamic acid (AHA) have been identified as suitable reagents for the control of Pu and Np in advanced separation processes for nuclear fuel reprocessing such as the Advanced PUREX or UREX based recycle processes, due to their ability to strip the tetravalent form of Pu and Np from tri-butyl phosphate into nitric acid. However, both free and metal bound hydroxamates are known to undergo acid catalysed hydrolysis at low pH, the kinetics of which must be characterised before implementation of PUREX/UREX based reprocessing flowsheets. In support of this implementation, a comprehensive thermodynamic and kinetic model that describes both the complex speciation and hydrolysis of AHA in the presence of Np(IV) has been developed. The model has two unique features: (i) in the case of the hydrolysis reaction kinetics, the model includes the hydrolysis of not only free AHA but also both the mono- and bishydroxamato-Np(IV) complexes; (ii) for the associated speciation calculations, the model explicitly includes the ionic strength dependence of not only the mono- and bishydroxamato-Np(IV) complexes but also the mono- and bisnitrato neptunium(IV) and monohydroxoneptunium(IV) complexes. For the latter three species, respective SIT coefficients of Δε1,NO3 = −0.13 ± 0.03 kg mol−1, 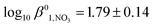 , Δε2,NO3 = −0.37 ± 0.13 kg mol−1,
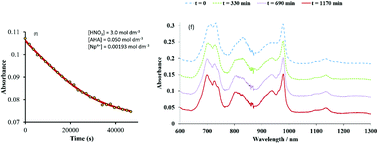
, Δε2,NO3 = −0.37 ± 0.13 kg mol−1, 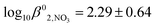 Δε1,OH = −0.36 kg mol−1 and log10 K01,OH = −1.23 were also determined. Using experimental data from a series of kinetic studies on the Np(IV)–AHA system, this model has been used to determine the rate constants for hydrolysis of mono– and bis–acetohydroxamatoneptunium(IV) at 25 °C for the first time. These were found to be 3.5 × 10−5 ± 2.5 × 10−5 dm3 mol−1 s−1 and 1.9 × 10−3 ± 1.3 × 10−3 dm3 mol−1 s−1, respectively. Comparison of these values with the rate constants for hydrolysis of free AHA indicates that complexation of AHA with Np(IV) increases the rate of hydroxamate hydrolysis – an observation that we attribute to the electron withdrawing effect of the metal centre within the Np(IV)–AHA complex increasing the susceptibility of the AHA carbonyl carbon to nucleophilic attack, the accepted first step in its mechanism of hydrolysis.
Δε1,OH = −0.36 kg mol−1 and log10 K01,OH = −1.23 were also determined. Using experimental data from a series of kinetic studies on the Np(IV)–AHA system, this model has been used to determine the rate constants for hydrolysis of mono– and bis–acetohydroxamatoneptunium(IV) at 25 °C for the first time. These were found to be 3.5 × 10−5 ± 2.5 × 10−5 dm3 mol−1 s−1 and 1.9 × 10−3 ± 1.3 × 10−3 dm3 mol−1 s−1, respectively. Comparison of these values with the rate constants for hydrolysis of free AHA indicates that complexation of AHA with Np(IV) increases the rate of hydroxamate hydrolysis – an observation that we attribute to the electron withdrawing effect of the metal centre within the Np(IV)–AHA complex increasing the susceptibility of the AHA carbonyl carbon to nucleophilic attack, the accepted first step in its mechanism of hydrolysis.


 Please wait while we load your content...
Please wait while we load your content...