Investigation of the Lewis acidic behaviour of an oxygen-bridged planarized triphenylborane toward amines and the properties of their Lewis acid–base adducts†
Abstract
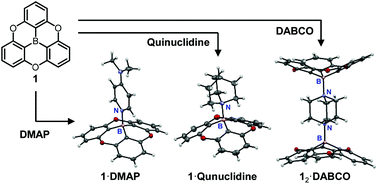
Lewis acid behavior of an oxygen-bridged triphenylborane (1) to amines and the properties of Lewis acid–base adducts of 1 with amines have been investigated. UV-vis titration and 11B NMR experiments showed the formation of Lewis acid–base adducts of 1 with pyridine, DMAP, quinuclidine, and DABCO, respectively (1·amine). X-ray crystallographic analysis revealed that the planar shape of 1 was converted to a bowl shape by the formation of 1·amine. Interestingly, 1·quinuclidine, 12·DABCO, and 1·DABCO exhibited dual emissions. Excitation spectra and photoluminescence decay time measurements suggest that the dual emissions were ascribed to two excited species, i.e., [1·amine]* and [1]* generated by photodissociation in the excited states.


 Please wait while we load your content...
Please wait while we load your content...