Cation-tuned synthesis of the A2SO4·SbF3 (A = Na+, NH4+, K+, Rb+) family with nonlinear optical properties†
Abstract
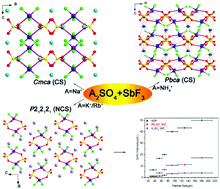
Four antimony fluoride sulfates named A2SO4·SbF3 (A = Na+, NH4+, K+, Rb+) have been successfully synthesized using a hydrothermal method by introducing Sb3+ cations with a stereochemically active lone pair in sulfates and subsequently tuning the structure through the second monovalent cations. All of the title compounds are stoichiometrically equivalent materials which share a common structural motif composed of a distorted SO4 tetrahedron and an asymmetric SbF3 polyhedron. However, the macroscopic centricities of these four compounds are significantly influenced by the size and coordination environment of cations; Na2SO4·SbF3 crystallizes in centrosymmetric space groups Cmca and (NH4)2SO4·SbF3 in Pbca, while K2SO4·SbF3 and Rb2SO4·SbF3 crystallizes in noncentrosymmetric space group P212121. Complete characterization including thermal analyses, infrared and UV-vis spectroscopy, and theoretical calculations is also reported. Powder second harmonic generation measurement for noncentrosymmetric K2SO4·SbF3 and Rb2SO4·SbF3 indicated that both of them are type I phase-matchable.


 Please wait while we load your content...
Please wait while we load your content...