Exceptionally slow magnetic relaxation in cobalt(ii) benzoate trihydrate†
Abstract
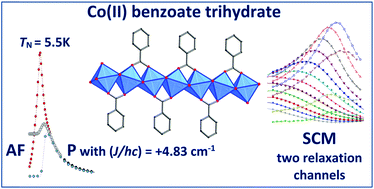
Cobalt(II) benzoate trihydrate prepared by the reaction of CoCO3 with benzoic acid (HBz) in boiling water followed by crystallization has been structurally characterized as a chain-like system with the formula unit [Co(Bz)(H2O)2]Bz·H2O where the Co(II) atoms are triply linked by one bridging syn–syn benzoato (Bz) and two aqua ligands; additional benzoate counter ions and solvate water molecules are present in the crystal structure. DC magnetic measurements reveal a sizable exchange coupling of a ferromagnetic nature between the Co(II) atoms. At TN = 5.5 K the paramagnetic phase switches to the antiferromagnetic phase. Though the remnant magnetization is zero, the magnetization curve shows two lobes of a hysteresis loop and the DC relaxation experiments confirm a long relaxation time at T = 2.0 K. AC susceptibility data confirm a slow relaxation of magnetization even in the antiferromagnetic phase. In the absence of the magnetic field, two relaxation channels exist. The relaxation time for the low frequency channel is as slow as τLF > 1.6 s and data fitting yields τLF (2.1 K) = 14 s. The high-frequency relaxation time obeys the Orbach process at a higher temperature whereas the Raman process dominates the low-temperature region. Three slow relaxation channels are evidenced at the applied magnetic field BDC = 0.1 T.


 Please wait while we load your content...
Please wait while we load your content...