Spin-crossover compounds based on iron(ii) complexes of 2,6-bis(pyrazol-1-yl)pyridine (bpp) functionalized with carboxylic acid and ethyl carboxylic acid†
Abstract
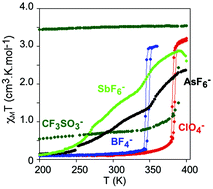
Four new salts of the iron(II) complex of the 2,6-bis(pyrazol-1-yl)pyridine ligand functionalized with a carboxylic acid group (bppCOOH) of formulas [Fe(bppCOOH)2](BF4)2 (1(BF4)2), [Fe(bppCOOH)2](CF3SO3)2·yMe2CO (1(CF3SO3)2·yMe2CO), [Fe(bppCOOH)2](AsF6)2·yMe2CO (1(AsF6)2·yMe2CO) and [Fe(bppCOOH)2](SbF6)2·yMe2CO (1(SbF6)2·yMe2CO) have been prepared and characterized together with a more complete structural and photomagnetic characterization of the previously reported [Fe(bppCOOH)2](ClO4)2 (1(ClO4)2). Furthermore, the iron(II) complex of the ethyl ester derivative of bppCOOH (bppCOOEt) has been prepared and characterized (compound [Fe(bppCOOEt)2](ClO4)2·yMe2CO, 2(ClO4)2·yMe2CO). Isostructural 1(BF4)2 and 1(ClO4)2 show an abrupt and reversible spin transition with a much lower T1/2 for the BF4− salt. CF3SO3−, SbF6− and AsF6− counteranions and the bppCOOEt ligand lead to the incorporation of solvent molecules in the structures, which play an important role in the spin-crossover properties of these compounds. In the case of 1(CF3SO3)2·yMe2CO, a reversible spin transition is obtained after desolvation. All these compounds show a LIESST effect.


 Please wait while we load your content...
Please wait while we load your content...