Monomeric nickel hydroxide stabilized by a sterically demanding phosphorus–nitrogen PN3P-pincer ligand: synthesis, reactivity and catalysis†
Abstract
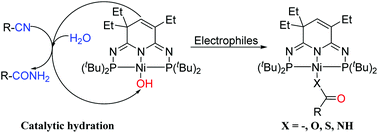
A terminal nickel hydroxide complex (PN3P)Ni(OH) (3) bearing the 2nd generation phosphorus–nitrogen PN3P-pincer ligand has been synthesized and structurally characterized. As a nucleophile, 3 reacts with CO to afford the hydroxycarbonyl complex 4, (PN3P)Ni(COOH). 3 can also activate CO2 and CS2 to produce nickel bicarbonate (PN3P)Ni(OCOOH) (5) and bimetallic dithiocarbonate [(PN3P)NiS]2CO (6) respectively, as well as to promote aryl isocyanate and isothiocyanate insertion into the Ni–OH bond to give the corresponding (PN3P)NiEC(O)NHAr complexes (E = O, 7; E = S, 8). In addition, 3 catalyzes the nitrile hydration to various amides with well-defined intermediates (PN3P)Ni–NHC(O)R (R = Me, 9; R = Ph, 10).


 Please wait while we load your content...
Please wait while we load your content...