Molybdenum(vi) tris(amidophenoxide) complexes†
Abstract
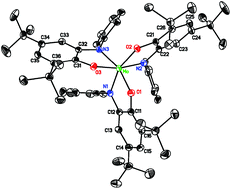
Tris(2-(arylamido)-4,6-di-tert-butylphenoxo)molybdenum(VI) complexes (Rap)3Mo can be prepared either from (cycloheptatriene)Mo(CO)3 and the N-aryliminoquinone, or from MoO2(acac)2 and the aminophenol. In contrast to all other reported unconstrained transition metal tris(amidophenoxide) complexes, the molybdenum complexes show a facial geometry in the solid state. In solution, the fac isomer predominates, though a small amount of mer isomer is detectable at room temperature. At elevated temperature the two species interconvert through Rây-Dutt trigonal twists, which are faster than Bailar twists in this system, presumably because of steric effects of the N-aryl groups. Substituents on the N-aryl ring shift the fac/mer equilibrium of the complex, with more electron-withdrawing substituents generally increasing the proportion of the mer isomer. The preference for fac over mer geometry is thus suggested to be due to enhanced π bonding in the fac isomer. In contrast to analogous catecholate complexes, the tris(amidophenoxide) complexes are not Lewis acidic and are inert to nucleophilic oxidants such as amine-N-oxides.


 Please wait while we load your content...
Please wait while we load your content...
