Electron-poor hemilabile dicationic palladium NHC complexes – synthesis, structure and catalytic activity†
Abstract
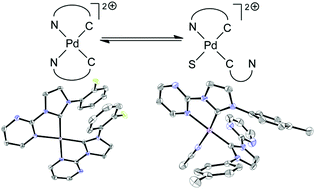
We present a new class of dicationic palladium NHC complexes with two carbene ligands bearing different aryl substituents and a hemilabile pyrimidyl group. They can either be synthesized from the imidazolium salts via the silver transmetalation route to a halide-free palladium(II) precursor or by direct deprotonation by palladium acetate. For four complexes we could determine their solid-state structures by X-ray diffraction experiments. Insight gained by NMR spectroscopy and DFT calculations revealed that in solution the two potentially bidentate ligands interchange between chelating and non-chelating coordination modes. The preference of these coordination modes is governed by the steric influence of the different ligands. The complexes were shown to be active catalysts for the hydroarylation of alkynes, and their performance was rationalized by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...