Post-synthetic modification of zirconium metal–organic frameworks by catalyst-free aza-Michael additions†
Abstract
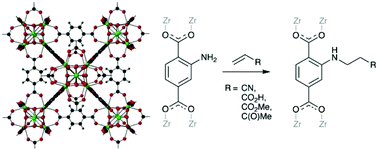
The reactions of the zirconium MOF [Zr6O4(OH)4(bdc-NH2)6] (UiO-66-NH2, bdc-NH2 = 2-amino-1,4-benzenedicarboxylate) with the Michael acceptors acrylonitrile (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN), acrylic acid (CH2
CHCN), acrylic acid (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2H), methyl acrylate (CH2
CHCO2H), methyl acrylate (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) and methyl vinyl ketone (CH2
CHCO2Me) and methyl vinyl ketone (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Me) led to post-synthetic modification of the MOF through C–N bond formation without loss of crystallinity. The reactions with acrylonitrile and acrylic acid go to completion, yielding [Zr6O4(OH)4(bdc-NHCH2CH2CN)6] (UiO-66-AN, 1) and [Zr6O4(OH)4(bdc-NHCH2CH2CO2H)6] (UiO-66-AA, 2) respectively, whereas those with methyl acrylate and methyl vinyl ketone are incomplete, yielding [Zr6O4(OH)4(bdc-NH2)0.66(bdc-NHCH2CH2CO2Me)5.34] (UiO-66-MA, 3) and [Zr6O4(OH)4(bdc-NH2)2.76(bdc-NHCH2CH2C(O)Me)3.24] (UiO-66-MVK, 4), respectively. The acrylonitrile-modified MOF UiO-66-AN undergoes further reaction with sodium azide in the presence of zinc(II) chloride in n-butanol to form the tetrazolate-modified MOF [Zr6O4(OH)4(bdc-NHCH2CH2CN)4.74(bdc-NHCH2CH2CN4H)1.26] (UiO-66-TZ, 5).
CHC(O)Me) led to post-synthetic modification of the MOF through C–N bond formation without loss of crystallinity. The reactions with acrylonitrile and acrylic acid go to completion, yielding [Zr6O4(OH)4(bdc-NHCH2CH2CN)6] (UiO-66-AN, 1) and [Zr6O4(OH)4(bdc-NHCH2CH2CO2H)6] (UiO-66-AA, 2) respectively, whereas those with methyl acrylate and methyl vinyl ketone are incomplete, yielding [Zr6O4(OH)4(bdc-NH2)0.66(bdc-NHCH2CH2CO2Me)5.34] (UiO-66-MA, 3) and [Zr6O4(OH)4(bdc-NH2)2.76(bdc-NHCH2CH2C(O)Me)3.24] (UiO-66-MVK, 4), respectively. The acrylonitrile-modified MOF UiO-66-AN undergoes further reaction with sodium azide in the presence of zinc(II) chloride in n-butanol to form the tetrazolate-modified MOF [Zr6O4(OH)4(bdc-NHCH2CH2CN)4.74(bdc-NHCH2CH2CN4H)1.26] (UiO-66-TZ, 5).


 Please wait while we load your content...
Please wait while we load your content...