Tuning the reactivity of copper complexes supported by tridentate ligands leading to two-electron reduction of dioxygen†
Abstract
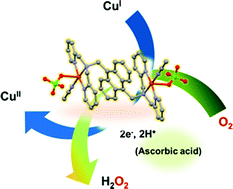
A series of copper complexes bearing polypyridyl tridentate ligands have been prepared to fine tune their reactivity toward the oxygen reduction reaction (ORR). During the process of preparation of our copper complexes, we successfully obtained two new crystal structures which are [Cu2(μ-Cl)2(adpa)2](ClO4)2 (2b) and [Cu2(addpa)(CH3CN)2(ClO4)2](ClO4)2 (3a) and a new structure [Cu2(addpa)(CH3CN)2(H2O)2](ClO4)4 (3b) captured after the catalytic ORR. Electrochemical studies and stoichiometric chemical reduction of copper(II) complexes by ascorbic acid indicated that the presence of an anthracene unit helps to facilitate the reduction of Cu(II) as well as the stabilisation of Cu(I) species. Regarding oxygen activation, the dinuclear Cu(I) complex 3a showed significantly higher ORR activity than its analogous mononuclear complex 2a. Complex 3a was also found to be relatively robust and competent in catalytic O2 reduction. The observed H2O2 product after this catalysis, together with the data obtained from DFT calculations supported that 3a exhibited a 2H+, 2e− catalytic activity towards the ORR as opposed to the expected 4H+, 4e− process usually found in copper complexes with tridentate ligands. The proton (H+) source for this process was expected from ascorbic acid which also serves as a reducing agent in this reaction. This work highlighted an approach for tuning the ORR activity of the copper complexes by the introduction of a conjugated-π moiety to the supporting ligand.


 Please wait while we load your content...
Please wait while we load your content...