Synthesis of heterobimetallic gold(i) ferrocenyl-substituted 1,2,3-triazol-5-ylidene complexes as potential anticancer agents†
Abstract
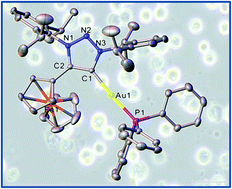
1,2,3-Triazol-5-ylidene (trz) complexes of gold(I) containing a ferrocenyl substituent on the C4-position of the trz ring were synthesized to yield the neutral heterobimetallic gold(I) trz chlorido (2), gold(I) trz phenyl (3), and the cationic gold(I) trz triphenylphospine (5) complexes. In order to compare the effect of silver(I) as central metal vs. gold(I), [Ag(trz)2]+ (4) was also prepared, while variation of the C4-1,2,3-triazol-5-ylidene substituent from a ferrocenyl to a phenyl group was done to prepare the monometallic analogue of 5, namely the cationic Au(I) trz triphenylphosphine complex 6. The complexes were characterised with spectroscopic and electrochemical methods, and the single crystal X-ray structures of 2–6 were determined. NMR stability studies of 5 as a representative example of the series of complexes were performed to confirm the stability of the complexes in the solvent dimethylsulfoxide and in aqueous solution. The anti-cancer potential of 5 was evaluated against the lung cancer cell lines A549 and H1975, and the human embryonic kidney cell line (HEK-293) was used as a non-cancer model. IC50 values of 0.89, 0.23 and 5.43 μM, respectively, were obtained for A549, H1975 and HEK-293, respectively, indicating the activity and selectivity of 5 for cancer cells. Fluorescence microscopy experiments as a preliminary mode-of-action study evidenced an apoptotic cell death mechanism rather than necrotic cell death.


 Please wait while we load your content...
Please wait while we load your content...