Synthesis of monofunctional platinum(iv) carboxylate precursors for use in Pt(iv)–peptide bioconjugates†
Abstract
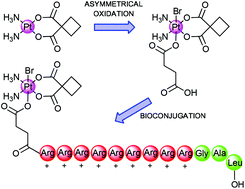
Herein we present platinum(IV) bioconjugates with polyarginine peptides as prospective prodrug delivery systems. Asymmetrical platinum(IV) complexes 3 were obtained via oxidation of parent platinum(II) complexes 2 with N-bromosuccinimide (NBS) in the presence of succinic anhydride. The combination of these two oxidation reagents furnishes the platinum(IV) environment with two different axial ligands, one of which bears a free carboxylic acid. All platinum(II) and (IV) compounds were characterized by FT-IR, ESI-MS, HPLC, 1H-, 13C- and 195Pt-NMR. Standard solid-phase peptide chemistry was used for the synthesis of polyarginine (R9) peptides. Coupling of the platinum complexes with peptides N-terminally afforded peptide monoconjugates, which were purified by semi-preparative HPLC and characterized by analytical HPLC and ESI-MS. Platinum(IV)–peptide bioconjugates as well as platinum(II) and platinum(IV) complexes were tested as cytotoxic agents against two different human cancer cell lines (MCF-7, HepG2) and normal human fibroblasts cell lines (GM5657T). Preliminary in vitro data showed that all platinum(IV) complexes exhibit lower activity than their platinum(II) precursors towards most cell lines. Interestingly, in the case of HepG2 cells, the Pt(IV)-(R)9-G-A-L bioconjugate (4a) showed even higher activity compared to the non-targeting platinum(IV) parent compound.


 Please wait while we load your content...
Please wait while we load your content...