The selective formation of a 1,2-disilabenzene from the reaction of a disilyne with phenylacetylene†‡
Abstract
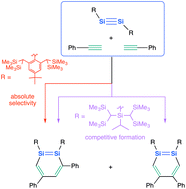
A stable 3,5-diphenyl-1,2-disilabenzene was selectively synthesized by the reaction between the isolable disilyne TbbSi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiTbb (Tbb = 2,6-[CH(SiMe3)2]2-4-t-Bu-phenyl) with phenylacetylenes. Its molecular structure and physical properties were examined and compared to those of the 1,2-disilabenzene that was obtained from the reaction between TbbSi
SiTbb (Tbb = 2,6-[CH(SiMe3)2]2-4-t-Bu-phenyl) with phenylacetylenes. Its molecular structure and physical properties were examined and compared to those of the 1,2-disilabenzene that was obtained from the reaction between TbbSi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiTbb and acetylene. Moreover, a plausible formation mechanism for this reaction is discussed.
SiTbb and acetylene. Moreover, a plausible formation mechanism for this reaction is discussed.


 Please wait while we load your content...
Please wait while we load your content...