Group 15 biradicals: synthesis and reactivity of cyclobutane-1,3-diyl and cyclopentane-1,3-diyl analogues
Abstract
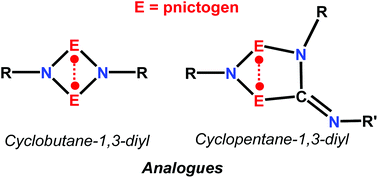
Readily accessible group 15 biradicals of the type [E(μ-NR)]2 (E = P–Bi) form planar 6π-electronic-4-membered heterocycles featuring open-shell singlet biradical character. They can be utilized to activate small molecules bearing single, double and triple bonds as well as to trap labile in situ generated fragments. In the reaction with CO and R–NC (R = small alkyl or aryl substituent) pnictogen analogues of cyclopentane-1,3-diyl are formed which represent robust molecular switches. Group 15 biradicals with two different radical sites display regioselectivity upon addition of small molecules.
- This article is part of the themed collection: 2018 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...