Generation of stannabenzenes and their monomer–dimer equilibration†
Abstract
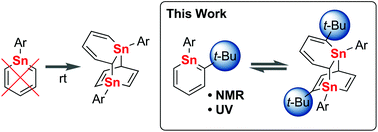
Stannabenzene has not been isolated so far because of its high reactivity. In order to synthesize and isolate a stable stannabenzene, we have introduced two steric protection groups, the Tbt (2,4,6-tris[bis(trimethylsilyl)methyl]phenyl) or the Bbt (2,6-bis[bis(trimethylsilyl)methyl]-4-[tris(trimethylsilyl)methyl]phenyl) group on the tin atom and the t-butyl group on the 2-position, to the stannabenzene skeleton. Such a combination of steric protection groups was found to suppress the dimerization of stannabenzene to some extent, giving an equilibrated mixture of the corresponding stannabenzene and its dimer.


 Please wait while we load your content...
Please wait while we load your content...