Effects of non-covalent interactions on the electronic and electrochemical properties of Cu(i) biquinoline complexes†
Abstract
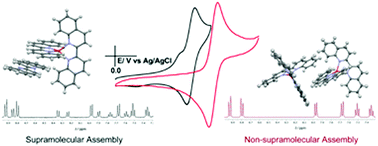
A Cu(I) complex {[CuI(biq)2]ClO4-biq} with biq = 2,2′-biquinoline was prepared, fully characterized and its properties compared with those of the well-known [CuI(biq)2]ClO4 complex. The crystal structures were obtained for both complexes (crystal structure for [CuI(biq)2]ClO4 has not been previously reported). Complex [CuI(biq)2]ClO4 crystallizes as a racemate where each enantiomer has a different τ4 value while compound {[CuI(biq)2]ClO4-biq} crystallizes as a non-chiral supramolecular aggregate with an uncoordinated biq molecule forming a π–π stacking interaction with a coordinated biq. 1H-NMR spectroscopy in non-coordinating solvents reveals that structures in solution are similar to those in the solid phase, confirming the presence of a supramolecular arrangement for compound {[CuI(biq)2]ClO4-biq}. The stability of the non-covalent aggregate in solution of {[CuI(biq)2]ClO4-biq} causes significant differences between the spectroscopic and electrochemical properties of {[CuI(biq)2]ClO4-biq} and [CuI(biq)2]ClO4.


 Please wait while we load your content...
Please wait while we load your content...
