Challenges in cyclometalation: steric effects leading to competing pathways and η1,η2-cyclometalated iridium(iii) complexes†
Abstract
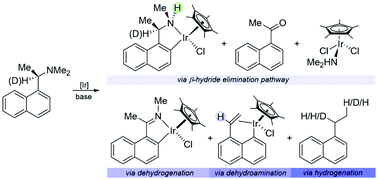
The iridation of (R)-N,N-dimethyl-1-(1-naphthyl)ethylamine in the presence of a base afforded an assortment of products ranging from organic molecules to coordinated systems and cyclometalated complexes. The transformation affirmed the postulation where steric effects within the coordination sphere favor a β-hydride elimination-like decomposition pathway, competing alongside ortho-metalation, thus leading to iminium intermediates. The same procedure also generated an unprecedented carbocyclic η1,η2-cycloiridated species that could not be attained from the direct cyclometalation of its organic ligand.


 Please wait while we load your content...
Please wait while we load your content...