The mechanism and origin of the regioselectivity of cobalt-catalyzed annulation of allenes with benzamide: a computational study†
Abstract
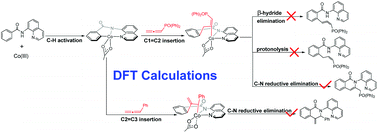
Thrimurtulu et al. recently reported unprecedented cobalt-catalyzed annulation of allenes with benzamide (N. Thrimurtulu, A. Dey, D. Maiti, C. M. R. Volla, Angew. Chem., Int. Ed., 2016, 55, 12361–12365). In this reaction, the substituent on the allene controls the regioselectivity for the formation of either dihydroisoquinolin-1(2H)-one or isoquinolin-1(2H)-one. In the present study, density functional theory calculations were performed to investigate the detailed reaction mechanism and the origin of the experimentally observed regioselectivity. A systematic search shows that the electronic and steric effects of the substituent on the allene determine which of the two allene insertions is followed, and thus determine the regioselectivity. The bulky diphenylphosphonate and two phenyl substituents of the allenylphosphonate and diarylallene favor C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2 insertion, which eventually leads to the formation of isoquinolin-1(2H)-one. In contrast, for the arylallene, which has a relatively electron-rich C2
C2 insertion, which eventually leads to the formation of isoquinolin-1(2H)-one. In contrast, for the arylallene, which has a relatively electron-rich C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 bond, C2
C3 bond, C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 insertion is favored and eventually leads to the formation of dihydroisoquinolin-1(2H)-one. The calculations also explain why annulation rather than hydroarylation of benzamide with allenylphosphonate occurs with a cobalt catalyst.
C3 insertion is favored and eventually leads to the formation of dihydroisoquinolin-1(2H)-one. The calculations also explain why annulation rather than hydroarylation of benzamide with allenylphosphonate occurs with a cobalt catalyst.


 Please wait while we load your content...
Please wait while we load your content...