Design of oxophilic metalloporphyrins: an experimental and DFT study of methanol binding†
Abstract
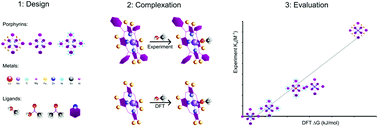
By systematic measurements we have evaluated a series of tetraphenyl metalloporphyrins and halogenated tetraphenyl metalloporphyrin derivatives for binding to ligands with oxygen containing functional groups, using methanol, acetic acid and acetone as examples. Experimental binding constants identified three metalloporphyrins with good binding to all three ligands: MgTPFPP, MgTPPBr8 and ZnTPPBr8 as well as a range of porphyrins binding to select ligands. Based on these results the optimal porphyrins can be selected for the desired binding interactions. We also show how to use DFT calculations to evaluate the potential binding between a metalloporphyrin and a ligand, which is deduced from free energies of binding ΔG, charge transfer ΔQ, and change of metal spin state. Computations on unsubstituted porphyrins in lieu of tetraphenyl porphyrin systems yield reliable predictions of binding interactions with good correlation to the corresponding experimental data. The calculations have also yielded interesting insights into the effect of halogenation in the β-position on the binding to ligands with oxygen containing functional groups.


 Please wait while we load your content...
Please wait while we load your content...