Neuroglobin is capable of self-oxidation of methionine64 introduced at the heme axial position†
Abstract
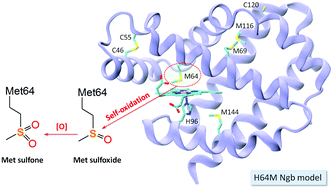
Neuroglobin (Ngb), with its physiological role not fully understood, was found to be capable of self-oxidation of methionine64 introduced at the heme axial position (H64M Ngb), adopting a high-spin heme state and producing both methionine sulfoxide (SO-Met) and sulfone (SO2-Met), which represents the structure and function of cytochrome c in a non-native state.


 Please wait while we load your content...
Please wait while we load your content...