The Heck reaction as a tool to expand polyoxovanadates towards thiol-sensitive organic–inorganic hybrid fluorescent switches†
Abstract
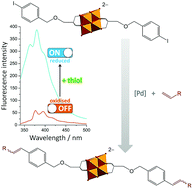
Among the Lindqvist-type hexavanadates synthesised herein by the palladium-catalysed Heck reactions a C–C cross-coupled organic–inorganic hybrid with the largest conjugated, stilbene-derivatised π ligands is reduced in the presence of thiols producing a remarkable increase in fluorescence intensity with respect to the fully-oxidised variant. This enhanced emission (ON-state) of the reduced species can be switched OFF if the solution is subjected to e.g. air where the species is re-oxidised.


 Please wait while we load your content...
Please wait while we load your content...