Redox transmetallation approaches to the synthesis of extremely bulky amido-lanthanoid(ii) and -calcium(ii) complexes†
Abstract
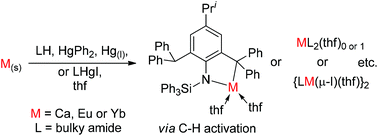
Redox transmetallation protolysis reactions between HgPh2, elemental metals, M = Ca, Eu, or Yb, and the bulkyl arylsilylamine PhL†H (HN(SiPh3)(Ar†), Ar† = C6H2Pri{C(H)Ph2}2-4,2,6), or the borylsilylamine PhLBoH (HN(SiPh3){B(DipNCH)2}, Dip = C6H3Pri2-2,6) pro-ligands yielded complexes incorporating doubly deprotonated, N,C-chelating amido/organyl ligands, viz. [M(L–H) (thf)x]n, L–H = PhL†–H (1–3) or PhLBo–H (4–5); M = Ca (1, 4), Eu (2) or Yb (3, 5); x = 0 (5) or 2 (1–4); n = 1 (1–4) or 2 (5). Structural differences between 4 and 5 represent a rare divergence in the chemistry of divalent calcium and ytterbium. Utilisation of a less hindered bis(aryl) amine, DLMH (HN(Dip)(Mes), Mes = C6H2Me3-2,4,6) in a similar reaction yielded a three-coordinate, trigonal planar ytterbium complex [Yb(DLM)2(thf)] (6). Direct redox transmetallation reactions between an amido-mercurial iodide [(MeL†)HgI] (7, MeL† = –N(SiMe3)(Ar†)) and ytterbium or europium metal afforded homoleptic [Yb(MeL†)2] (8) and heteroleptic [{Eu(MeL†)(μ-I)(thf)}2] (9) respectively. This study highlights the versatility of redox transmetallation pathways to amido-lanthanoid complexes, especially where such compounds are difficult to access using conventional salt metathesis pathways.


 Please wait while we load your content...
Please wait while we load your content...
