Bis(oxazoline)-derived N-heterocyclic carbene ligated rare-earth metal complexes: synthesis, structure, and polymerization performance†
Abstract
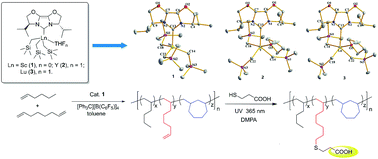
Bis(oxazoline)-derived N-heterocyclic carbene (IBiox) supported rare-earth (Sc, Y, Lu) trialkyl complexes have been synthesized and structurally characterized, and their catalytic activity in the (co)polymerization of α-olefins has been studied. The treatment of Ln(CH2SiMe3)3(THF)2 with one equivalent of freshly prepared IBiox afforded the rare-earth metal complexes (IBiox)Ln(CH2SiMe3)3THFn (Ln = Sc (1), n = 0; Y (2), n = 1; and Lu (3), n = 1) in good yields. Single crystal X-ray diffraction study showed that 1 is pseudo tetrahedral, while 2 and 3 are distorted trigonal bipyramidal with coordinated THF. The Ln–C(carbene) bond distances in 1, 2, and 3 are 2.352, 2.550, and 2.479 Å, respectively. DFT calculations were performed to study the bonding scheme and the structural stability. Complex 1 showed a high activity for 1-hexene polymerization by activation with 2 equivalents of [Ph3C][B(C6F5)4], and the resultant polymers are predominantly vinylene end groups (ca. 95%). Moreover, the catalyst system based on 1 proved to be effective for the copolymerization of 1-hexene with 1,7-octadiene, affording the copolymers with about 20% pendant vinyl groups. The hydrophilicity of the copolymers was improved by modifying the vinyl groups with carboxyls via a thiol–ene reaction.


 Please wait while we load your content...
Please wait while we load your content...