Effects of diminished steric protection at phosphorus on stability and reactivity of oxaphosphirane complexes†
Abstract
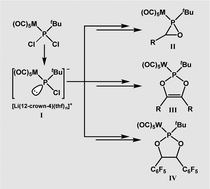
Readily available P-tBu substituted Li/Cl phosphinidenoid complexes react with carbonyl compounds to furnish sterically almost unhindered oxaphosphirane complexes that reveal new and surprisingly facile intra- and intermolecular ring expansion reactions. 1,3,2-Dioxaphosphole complex formation is explained by DFT calculations through diastereoselective carbonyl group-induced ring cleavage of an oxaphosphirane intermediate.


 Please wait while we load your content...
Please wait while we load your content...