Why nature chose the Mn4CaO5 cluster as water-splitting catalyst in photosystem II: a new hypothesis for the mechanism of O–O bond formation
Abstract
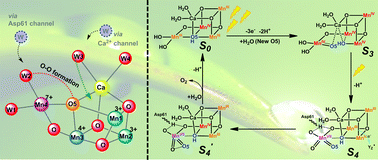
Resolving the questions, namely, the selection of Mn by nature to build the oxygen-evolving complex (OEC) and the presence of a cubic Mn3CaO4 structure in OEC coupled with an additional dangling Mn (Mn4) via μ-O atom are not only important to uncover the secret of water oxidation in nature, but also essential to achieve a blueprint for developing advanced water-oxidation catalysts for artificial photosynthesis. Based on the important experimental results reported so far in the literature and on our own findings, we propose a new hypothesis for the water oxidation mechanism in OEC. In this new hypothesis, we propose for the first time, a complete catalytic cycle involving a charge-rearrangement-induced MnVII–dioxo species on the dangling Mn4 during the S3 → S4 transition. Moreover, the O–O bond is formed within this MnVII–dioxo site, which is totally different from that discussed in other existing proposals.
- This article is part of the themed collection: 2018 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...