Retention and multiphase transformation of selenium oxyanions during the formation of magnetite via iron(ii) hydroxide and green rust†
Abstract
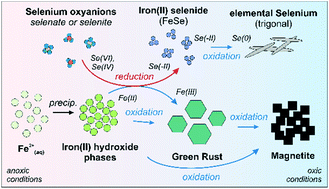
Environmental and health hazards associated with the trace element selenium are mainly related to the presence of the highly mobile selenium oxyanions selenite and selenate (oxidation states IV and VI). In this study, we investigated the immobilization of dissolved selenite and selenate during the formation of magnetite in coprecipitation experiments based on the progressive oxidation of an alkaline, anoxic Fe2+ system (pH 9.2). Up to initial selenium concentrations of 10−3 mol L−1 (mass/volume ratio = 3.4 g L−1), distribution coefficient values (log Kd) of 3.7 to 5.1 L kg−1 demonstrate high retention of selenium oxyanions during the mineral formation process. This immobilization is due to the reduction of selenite or selenate, resulting in the precipitation of sparingly soluble selenium compounds. By X-ray diffraction analysis, these selenium compounds were identified as trigonal elemental selenium that formed in all coprecipitation products following magnetite formation. Time-resolved analysis of selenium speciation during magnetite formation and detailed spectroscopic analyses of the solid phases showed that selenium reduction occurred under anoxic conditions during the early phase of the coprecipitation process via interaction with iron(II) hydroxide and green rust. Both minerals are the initial Fe(II)-bearing precipitation products and represent the precursor phases of the later formed magnetite. Spectroscopic and electron microscopic analysis showed that this early selenium interaction leads to the formation of a nanoparticulate iron selenide phase [FeSe], which is oxidized and transformed into gray trigonal elemental selenium during the progressive oxidation of the aquatic system. Selenium is retained regardless of whether the oxidation of the unstable iron oxides leads to the formation of pure magnetite or other iron oxide phases, e.g. goethite. This reductive precipitation of selenium induced by interaction with metastable Fe(II)-containing iron oxide minerals has the potential to influence the mobility of selenium oxyanions in contaminated environments, including the behavior of 79Se in the near-field of nuclear waste repositories.


 Please wait while we load your content...
Please wait while we load your content...